Article
Magnesium (MgYREZr) bioabsorbable screws in orthopedic surgery
A narrative review
Abstract
Magnesium-based bioabsorbable screws are a relatively new member of the family of orthopedic fixation materials. This new biomaterial will be fully absorbed, supports fracture union via osteoconductive capacities and potentially prevents infection. Among these implants, MgYREZr is the first approved alloy for clinical use in orthopedic surgery and has been in clinical practice since 2013. However, there are a limited number of clinical studies reporting the functional and radiological outcomes, indications and complications of these materials, and there is no extensive review article from the surgeon’s point of view. In this narrative review clinical studies, radiological findings, indications, and outcomes of magnesium bioabsorbable screws are discussed.
1. Introduction
Currently, most of the fracture fixation materials used in orthopedic surgery are made of metallic implants such as stainless steel, titanium, and cobalt-chromium-based alloys. Although these materials have high mechanical strength and stability, they induce stress shielding in the long term due to the difference in elastic modulus between the native bone and these materials. Furthermore, these metallic implants may necessitate removal late after fracture union secondary to residual pain, irritation or inflammatory reactions [1]. Implant removal operations have several disadvantages in various aspects. This second operation creates a significant burden for the limited health care sources [2]. Also, implant removal is prone to several complications. Serious complications such as re-fracture, infection, nerve, and vascular injuries may occur during the dissection of fibrotic tissues [3]. Furthermore, the absence of a second surgery is of significant contribution to the psychology of patients.
For the reasons mentioned above, many studies have been performed, and alternative bioabsorbable materials and products have been developed which can be used instead of permanent metallic implants in the history of orthopedic surgery. The ideal fracture fixation material should initially have appropriate mechanical strength, should be biocompatible, should not have adverse effects on fracture union, and should be entirely absorbed after fracture union has been completed. For this purpose, biomaterials made of various polymers, such as polyglycolic acid or polylactic acid, have been developed and used for many years in orthopedic surgery. However, low mechanical strength and load-bearing capacity limited their indications [4], and some problems have been encountered such as inflammatory and foreign body reactions [5].
In search of finding the optimal bioabsorbable biomaterial, research was directed towards the metals that can physiologically be metabolized in the body. Some unique properties of magnesium (Mg) make it an attractive biomaterial for manufacturing orthopedic implants. First of all, the density and the elastic modulus of Mg are very close to cortical bone compared to other metals such as titanium [6]. Moreover, degradation products are non-toxic, and they even induce or promote new bone formation and provide resistance to infection [7, 8]. Therefore, a huge number of preclinical studies focused on Mg and its alloys. Orthopedic implants made of Mg have been produced and presented to the market within this decade.
Mg is a well-known biomaterial, and its first use in orthopedic surgery was in the early 1900s. Since the first implants were produced from pure Mg that underwent a rapid degradation, the results obtained were not as desired. Rapid degradation caused a rapid loss of biomechanical properties and the emergence of a large amount of degradation products within tissues, particularly gas [9]. The problem was the timing of degradation. However, advances in biomaterial science, manufacturing techniques, and surface treatments optimized the degradation process in new generation Mg alloys such as MgYREZr. In other words, the degradation time in vivo is extended, allowing the implants to maintain their biomechanical stability for a longer time in the tissue, and the amount of degradation products that were released within in a certain period was reduced [10, 11]. These implants were programmed to vanish just after they completed their function, and they deserve to be called “smart implants”. This new generation of Mg alloy implants entered the market in 2013 (as compression screws) after they have been clinically approved and obtained CE mark, and have been in use since then [12].
Since this Mg-based bioabsorbable screw is a relatively new product in orthopedic surgery, there are few clinical studies on a limited number of patients. There is no extensive review of the clinical results and complications of this biomaterial in current literature. On the other hand, the use of these implants in orthopedic surgery has been increasing in recent years due to their potential to eradicate secondary implant removal operations. This manuscript aims to provide a narrative literature review regarding the role of magnesium bioabsorbable screws in orthopedic surgery, indications, clinical results, imaging findings, and complications.
2. Indications of Magnesium Bioabsorbable Screws
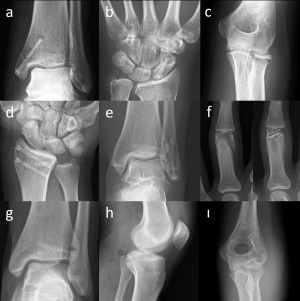 Figure 1. Representative cases to the indications of magnesium screws. (a) Medial malleolar fracture (b) Scaphoid fracture (c) Radial head fracture (d) Distal radius fracture (e) Lateral malleolar fracture (f) Phalangeal fracture (g) Adolescent Tillaux fracture (h) Posterior cruciate ligament avulsion fracture (ı) Humerus capitellum fracture
Figure 1. Representative cases to the indications of magnesium screws. (a) Medial malleolar fracture (b) Scaphoid fracture (c) Radial head fracture (d) Distal radius fracture (e) Lateral malleolar fracture (f) Phalangeal fracture (g) Adolescent Tillaux fracture (h) Posterior cruciate ligament avulsion fracture (ı) Humerus capitellum fracture
The Mg screw (MAGNEZIX® CS) can be used in a variety of indications in orthopedic and trauma surgery. Basically, fractures and non-unions of small bones and small bone arthrodesis, including, but not limited to, scaphoid fractures, avulsion fractures, medial and lateral malleolar fractures, intra-articular fractures of the tarsals, metatarsals, carpals and metacarpals, bunionectomy and osteotomies around the foot and ankle, arthrodesis of small joints (e.g. phalanges); fractures of patella, ulna and radial styloid fractures, radial head fractures, distal humerus intraarticular fractures [13].
3. Implants and Surgical Technique
MAGNEZIX® CS screws are manufactured in a Herbert screw design
 Figure 2. The appearance of the MAGNEZIX® 3.2 mm Ø compression screw
Figure 2. The appearance of the MAGNEZIX® 3.2 mm Ø compression screw
. Herbert screw is a variable pitch, headless, cannulated compression screw, which is invented by Timothy James Herbert who is a British orthopedic surgeon specializing in hand surgery. It is developed for stable compressive osteosynthesis of scaphoid fractures [14].
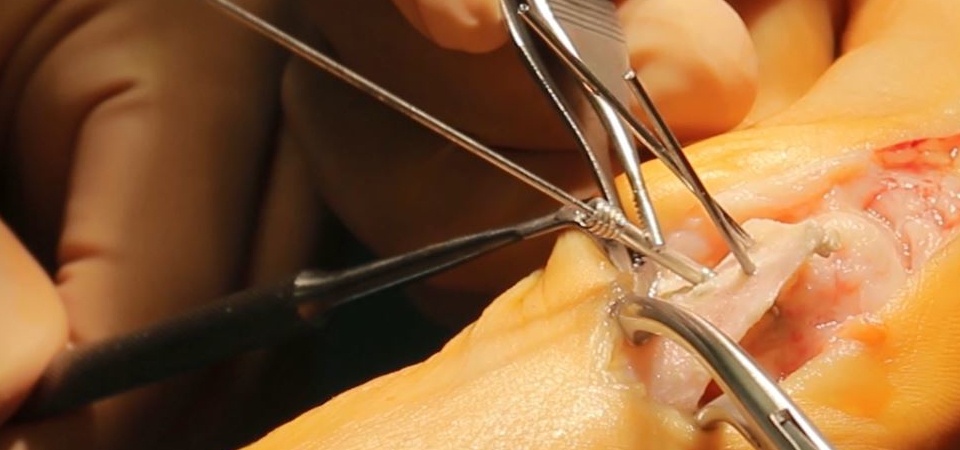
These screws have some unique properties that provide significant advantages and conveniences during surgery. First, headless means that the screw is completely embedded in the bone, without any protrusions to cause tissue irritation even in intra-articular placement. Cannulation provides proper placement of the screws and also allows for percutaneous application. Unlike, standard cortical screws which have constant screw pitch, these screws offer interfragmentary compression because of the pitch difference between the proximal and distal threads. Compression secures the fracture stability and ensures a more favorable biological environment for fracture union.
The application and handling of magnesium screws are quite similar to those of titanium and steel implants. Like standard cannulated screw application, first the pilot hole is drilled with a cannulated drill over a temporary guide K-wire; afterward the main hole is drilled, and finally, the screw is inserted in the desired position. Since magnesium is a “soft metal” in comparison with a screwdriver, it is essential not to apply excessive torque during screwing to ensure that the screw head is not peeled off.
4. Imaging findings of magnesium bioabsorbable screws
During the degradation process, in other words: the absorption period, of Mg implants, some ‘unusual imaging findings’ may be observed. These imaging findings are in fact component of this normal degradation process. However, many orthopedic surgeons are not familiar with these findings. Herein, the findings in direct radiography, computerized tomography (CT) and magnetic resonance imaging (MRI) are discussed in detail.
4.1. Direct Radiography
Direct radiography is the most commonly used radiological modality in orthopedic surgery, both for diagnosis and follow-up for fracture treatment. The surgeons often decide the union of fracture through physical examination findings such as lack of pain, the ability of painless weight bearing, and return to normal function together with radiological findings such as callus formation, the disappearance of the fracture line and consolidation of bone. Thus, radiographic examination is a vitally important tool for orthopedic surgeons [15]. However, magnesium-based implants demonstrate uncommon, so to say ‘unconventional’ radiographic findings which may cause incorrect interpretation. Immediately after the implantation of Mg screws to the bone, the process of degradation (in other words: corrosion) starts. Interaction between Mg and body fluids results in MgOH (magnesium hydroxide) and H2 gas formation [16].
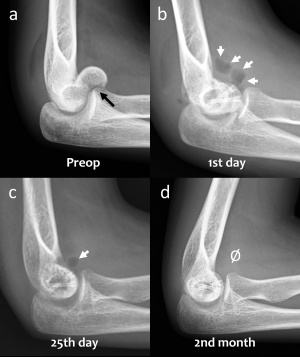 Figure 3. (a) Preoperative lateral elbow radiograph showing (black arrow) the coronal fracture of the distal humerus at capitellum. (b) Elbow radiograph shows gas within the soft tissue (white arrows) at the first postoperative day. (c) Elbow radiograph at the 25th postoperative day shows a diminished volume of gas (white arrow). (d) No gas is seen within the soft tissue at the 2nd month radiographic follow-up.
Figure 3. (a) Preoperative lateral elbow radiograph showing (black arrow) the coronal fracture of the distal humerus at capitellum. (b) Elbow radiograph shows gas within the soft tissue (white arrows) at the first postoperative day. (c) Elbow radiograph at the 25th postoperative day shows a diminished volume of gas (white arrow). (d) No gas is seen within the soft tissue at the 2nd month radiographic follow-up.
During the early postoperative radiographs, gas can be observed in the soft tissues that are distributed around the anatomic tissue planes or the tissue planes formed by surgical dissection. Clinically, however, this gas does not produce a palpable crepitation from the skin or any other symptoms. The gas shadows in the soft tissue usually disappear rapidly. Gas within the soft tissues, in early postoperative radiographs, is an alerting finding that can confuse with gas-producing anaerobic infections (with conventional implants). The formation of gas during degradation of Mg implants is a completely different entity – it is neither a loosening of the implant nor an infection.
Similarly, the gas usually begins to be apparent around the screws within the first three months. Because the degradation process continues, the amount of gas increases and spreads within the trabecular bone. Unlike soft tissues, gas within the bone is resorbed in a longer time. However, the gas is totally absorbed, and the Mg screws can be seen as a silhouette in the long term . In animal studies, it has been shown that screw is replaced by the cortical bone (7).
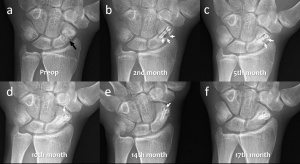 Figure 4. Serial wrist radiographs of a 19-year-old male patient with scaphoid pseudoarthrosis treated with magnesium screw fixation. (a) Preoperative postero-anterior (PA) wrist radiograph showing the scaphoid pseudoarthrosis (black arrow). (b-f) Serial PA wrist radiographs demonstrate the gradual absorption of gas within the bone at 2nd, 5th, 10th, 14th, and 17th follow-up. Note the union and consolidation of the pseudoarthrosis.
Figure 4. Serial wrist radiographs of a 19-year-old male patient with scaphoid pseudoarthrosis treated with magnesium screw fixation. (a) Preoperative postero-anterior (PA) wrist radiograph showing the scaphoid pseudoarthrosis (black arrow). (b-f) Serial PA wrist radiographs demonstrate the gradual absorption of gas within the bone at 2nd, 5th, 10th, 14th, and 17th follow-up. Note the union and consolidation of the pseudoarthrosis.
4.2. Computerized Tomography
Mg screws have similar CT imaging findings to that of the radiographic findings regarding gas formation and absorption. In the early period, both the screw and gas accumulations around the screw can be observed clearly
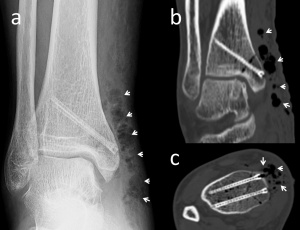 Figure 5. 53-year-old female who underwent medial malleolar osteotomy and mosaicplasty for the treatment of talus osteochondral lesion. The biplanar medial malleolar osteotomy was fixed with two magnesium screws. (a) Anteroposterior ankle radiograph at the 7th postoperative day shows diffuse gas (white arrows) in the soft tissues at the surgical site. (b) Coronal and (c) axial CT images both showing the gas (white arrows).
Figure 5. 53-year-old female who underwent medial malleolar osteotomy and mosaicplasty for the treatment of talus osteochondral lesion. The biplanar medial malleolar osteotomy was fixed with two magnesium screws. (a) Anteroposterior ankle radiograph at the 7th postoperative day shows diffuse gas (white arrows) in the soft tissues at the surgical site. (b) Coronal and (c) axial CT images both showing the gas (white arrows).
. In long-term CT examination, it is noted that the gas has been absorbed and disappeared. As the screws turn into “cortical bone” they can still be seen as a silhouette . Adil et al. showed that these silhouettes had similar Hounsfield scale measurements to the adjacent cortical bone in tomographic density evaluation four years after implantation (17). Furthermore, compared to conventional metallic implants such as titanium screws, magnesium-based implants create minimal metallic artifacts (18-20). This provides a significant advantage during the follow-up of patients.
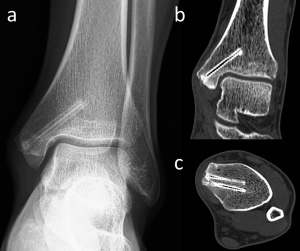 Figure 6. 24-year-old male with medial malleolar fracture treated with two magnesium screw fixation. (a) Anteroposterior ankle radiograph at the 30th-month follow-up shows the union of fracture and the silhouette of the screws. (b) Coronal and (c) axial CT images both show no gas within the bone and the screws show similar density with the adjacent cortical bone.
Figure 6. 24-year-old male with medial malleolar fracture treated with two magnesium screw fixation. (a) Anteroposterior ankle radiograph at the 30th-month follow-up shows the union of fracture and the silhouette of the screws. (b) Coronal and (c) axial CT images both show no gas within the bone and the screws show similar density with the adjacent cortical bone.
4.3. Magnetic Resonance Imaging
MRI scanners use strong magnetic fields and produce digital images utilizing the detectable radio frequency signals that are generated by the atoms within the tissues. On the other hand, when metals are placed in a magnetic field, such as a patient with metallic hardware, they tend to distort the magnetic field. These distortions significantly disrupt spatial encoding mechanisms used in conventional MRI and result in the images with artifacts and distorted anatomy. This is one of the problems that restrict the optimal evaluation of MRI in patients with metallic hardware (18-20).
Because magnesium (Mg) is one of the metals of Group IIa of the periodic table, it has also got certain ferromagnetic characteristics. However, previous experimental studies have shown that artifacts produced by magnesium implants are much less than conventional metals
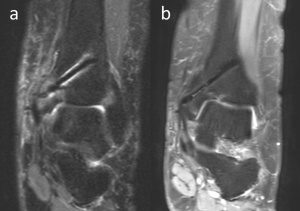 Figure 7. 54-year-old female patient with talus osteochondral lesion of the talus who was treated with medial malleolar osteotomy and mosaicplasty. PD weighted coronal MRI at 3rd (a) and 13th (b) months shows a decreasing amount of an artifact with time.
Figure 7. 54-year-old female patient with talus osteochondral lesion of the talus who was treated with medial malleolar osteotomy and mosaicplasty. PD weighted coronal MRI at 3rd (a) and 13th (b) months shows a decreasing amount of an artifact with time.
. Sonnow et al. compared the size of the artifacts produced by magnesium and titanium screws implanted to the chicken legs in 1.5 T MRI with different sequences. They reported that magnesium implants generate significantly fewer artifacts compared to titanium screws in MRI (18). Similarly, Filli et al. and Ernstberger et al. demonstrated less artifact formation in MRI with magnesium-based implants (19, 20). However, all of these studies are performed with non-corroded magnesium implants in either cadaveric specimens or agar. There is only one MRI study conducted in the 3rd year after implantation. Plaass et al. evaluated eight patients with MRI late after hallux valgus correction osteotomy and, reported a linear hypointensity, outlining the former implant site with no metal artifacts in patients (21). These findings indicate that the amount of artifact decreases over time following the absorption of the implant.
5. Clinical outcomes and complications
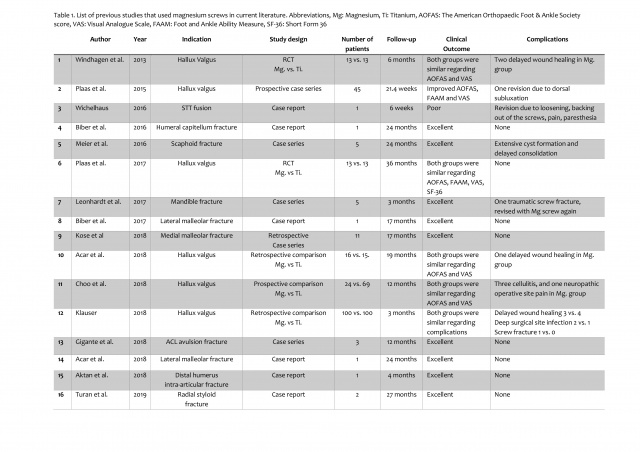
A total of 16 clinical studies and case reports have been reported up to date with the introduction of magnesium implants into clinical use in 2013
 9. Tables
Table 1. List of previous studies that used magnesium screws in current literature. Abbreviations, Mg: Magnesium, Ti: Titanium, AOFAS: The American Orthopaedic Foot & Ankle Society score, VAS: Visual Analogue Scale, FAAM: Foot and Ankle Ability Measure, SF-36: Short Form 36
9. Tables
Table 1. List of previous studies that used magnesium screws in current literature. Abbreviations, Mg: Magnesium, Ti: Titanium, AOFAS: The American Orthopaedic Foot & Ankle Society score, VAS: Visual Analogue Scale, FAAM: Foot and Ankle Ability Measure, SF-36: Short Form 36
(12, 17,21-34). Mg screws were used in metatarsal osteotomy fixation in hallux valgus in six of these previous studies (21-23, 29-31). Similar functional results were obtained in comparison with titanium screws, and the complication rates were similar. Thus, it has been shown that magnesium screws were not inferior to titanium screws in hallux valgus deformity correction. Successful results were also obtained in the medial and lateral malleolar fractures of the ankle without any nonunion case (27, 33). Kose et al. stated that the use of these screws would be advantageous especially in malleolar fractures where the need for implant removal is high (28). There are also reports on intraarticular use of magnesium screws. Gigante et al. used magnesium screws to fix anterior cruciate ligament avulsion fractures and reported excellent results (32). Aktan et al. used these screws to fix small intraarticular osteochondral fragments in comminuted distal humeral fractures for the restoration of the articular surface (34). Magnesium screws are also suitable for maxillofacial surgical applications (26).
Besides these favorable results, poor results have been reported in two studies (24, 25). In the first study, scapho-trapezio-trapezoid (STT) fusion was performed using two magnesium screws in a 42-year-old patient with a scaphoid fracture and STT arthritis. During the early period, extensive gas formation, loosening and cystic formation within the carpal bones were detected, and the patient underwent revision surgery (24). However, an operation failure was foreseeable since most likely several contraindications had been ignored. The second poor result was reported in acute scaphoid fracture fixation. Similar to the previous report, the authors observed extensive gas formation around the screw in the early post-operative period and evaluated these images as osteolysis and failure. However, clinical results were excellent at the final follow-up without any further intervention: all patients had an average value of 99 for the Mayo Wrist Score, and no nonunion occurred (25). At least, in the latter study, the misconception of the unique images mentioned in the previous section was obviously the reason for the unfavorable conclusion. Therefore, this shows the importance of accurate evaluation of radiological findings during the degradation period. The orthopedic surgeons who will use this implant should correctly evaluate these images and should be familiar with the corrosion of Mg-based biomaterials.
6. Conclusions
Orthopedic surgery has changed over the years not only with the description of new surgical techniques but also with new inventions of implant materials and design. The almost complete transition from stainless steel implants to titanium was the reality experienced by every orthopedic surgeon. Although this transition is yet to begin for Mg implants, it is likely that shortly the era of conventional implants will end. Similar functional outcomes with titanium implants have been achieved in a limited number of studies in the last six years. Only one case report reported a poor outcome. We think that this poor result is associated with an incorrect indication in the clinical situation. The bad summary in a case series of 5 patients was obviously related to a misinterpretation of the radiological findings. Thus, the correct description of these images and distinguishing between normal pathological processes and normal degradation processes is essential for both orthopedic surgeons and radiologists. Magnesium-based implants seem to find widespread use in many indications of fracture fixation compared to conventional metallic implants due to their significant advantages.
7. References
- Hofmann GO. Biodegradable implants in traumatology: a review on the state-of-the-art. Arch Orthop Trauma Surg. 1995;114(3):123-32.
- Böstman O, Pihlajamäki H (1996) Routine implant removal after fracture surgery: a potentially reducible consumer of hospital resources in trauma units. J Trauma 41(5):846–849.
- Kasai T, Matsumoto T, Iga T, Tanaka S. Complications of implant removal in ankle fractures. J Orthop. 2019;16(3):191-194. doi: 10.1016/j.jor.2019.02.017.
- Marukawa E, Tamai M, Takahashi Y, Hatakeyama I, Sato M, Higuchi Y, Kakidachi H, Taniguchi H, Sakamoto T, Honda J, Omura K, Harada H. Comparison of magnesium alloys and poly-l-lactide screws as degradable implants in a canine fracture model. J Biomed Mater Res B Appl Biomater.2016;104(7):1282-9. doi: 10.1002/jbm.b.33470.
- Raikin SM, Ching AC. Bioabsorbable fixation in foot and ankle. Foot Ankle Clin. 2005 Dec10(4):667-84, ix. Review.
- Kamrani S, Fleck C. Biodegradable magnesium alloys as temporary orthopaedic implants: a review. Biometals. 2019;32(2):185-193. doi: 10.1007/s10534-019-00170-y.
- Waizy H, Diekmann J, Weizbauer A, Reifenrath J, Bartsch I, Neubert V, Schavan R, Windhagen H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J Biomater Appl. 2014 Jan;28(5):667-75. doi: 10.1177/0885328212472215.
- Rahim MI, Eifler R, Rais B, Mueller PP. Alkalization is responsible for antibacterial effects of corroding magnesium. J Biomed Mater Res A. 2015 Nov;103(11):3526-32. doi: 10.1002/jbm.a.35503.
- Payr E. Beiträge zur Technik der Blutgefäss- und Nervennaht nebst Mitteilungen über die Verwendung eines resorbierbaren Metalles in der Chirurgie. Arch Klin Chir. 1900;62:67–93.
- Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010 May;6(5):1680-92. doi: 10.1016/j.actbio.2010.02.028.
- Liu C, Ren Z, Xu Y, Pang S, Zhao X, Zhao Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning. 2018 Mar 13;2018:9216314. doi: 10.1155/2018/9216314.
- Biber R, Pauser J, Geßlein M, Bail HJ (2016) Magnesium-Based Absorbable Metal Screws for Intra-Articular Fracture Fixation. Case Rep Orthop 2016:9673174.
- Seitz JM, Lucas A, Kirschner M (2016) Magnesium-based compression screws: a novelty in the clinical use of implants. JOM 68:1177-1182.
- Herbert TJ, Fisher WE. Management of the fractured scaphoid using a new bone screw. J Bone Joint Surg Br. 1984 Jan;66(1):114-23.
- Dijkman BG, Sprague S, Schemitsch EH, Bhandari M. When is a fracture healed? Radiographic and clinical criteria revisited. J Orthop Trauma. 2010 Mar;24 Suppl 1:S76-80. doi: 10.1097/BOT.0b013e3181ca3f97.
- Mueller WD, Nascimento ML, de Mele MFL (2010) Critical discussion of the results from different corrosion studies of Mg and Mg alloys for biomaterial applications. Acta Biomater 6:1749–1755.
- Turan A, Kati YA, Acar B, Kose O. Magnesium Bioabsorbable Screw Fixation of Radial Styloid Fractures: Case Report. Jnl Wrist Surg 2019 . DOI: 10.1055/s-0039-1685489.
- Sonnow L, Könneker S, Vogt PM, Wacker F, von Falck C. Biodegradable magnesium Herbert screw - image quality and artifacts with radiography, CT and MRI. BMC Med Imaging. 2017 Feb 14;17(1):16. doi: 10.1186/s12880-017-0187-7.
- Filli L, Luechinger R, Frauenfelder T, Beck S, Guggenberger R, Farshad-Amacker N, Andreisek G. Metal-induced artifacts in computed tomography and magnetic resonance imaging: comparison of a biodegradable magnesium alloy versus titanium and stainless steel controls. Skeletal Radiol. 2015 Jun;44(6):849-56. doi: 10.1007/s00256-014-2057-5.
- Ernstberger T, Buchhorn G, Heidrich G. Artifacts in spine magnetic resonance imaging due to different intervertebral test spacers: an in vitro evaluation of magnesium versus titanium and carbon-fiber-reinforced polymers as biomaterials. Neuroradiology. 2009 Aug;51(8):525-9. doi: 10.1007/s00234-009-0537-4.
- Plaass C, von Falck C, Ettinger S et al. (2017) Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies - 3 year results of a randomized clinical trial. J Orthop Sci. pii: S0949-2658(17)30300-7. https://doi.org/10.1016/j.jos.2017.11.005.
- Windhagen H, Radtke K, Weizbauer A et al. (2013) Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed Eng Online. 12:62. https://doi.org/ 10.1186/1475-925X-12-62.
- Plaass C, Ettinger S, Sonnow L et al. (2016) Early results using biodegradable magnesium screw for modified chevron osteotomies. J Orthop Res 34(12):2207-2214. https://doi.org/10.1002/jor.23241.
- Wichelhaus A, Emmerich J, Mittlmeier T (2016) A Case of Implant Failure in Partial Wrist Fusion Applying Magnesium-Based Headless Bone Screws. Case Rep Orthopv2016:7049130.
- Meier R, Panzica M (2017) First results with a resorbable MgYREZr compression screw in unstable scaphoid fractures show extensive bone cysts. Handchir Mikrochir Plast Chir 49(1):37-41.
- Leonhardt H, Franke A, McLeod NMH, Lauer G, Nowak A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br J Oral Maxillofac Surg. 2017 Jul;55(6):623-625. doi: 10.1016/j.bjoms.2017.04.007.
- Biber R, Pauser J, Brem M, Bail HJ (2017) Bioabsorbable metal screws in traumatology: A promising innovation. Trauma Case Reports (8):11-15.
- Kose O, Turan A, Unal M, Acar B, Guler F. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: short-term clinical and radiological outcomes in eleven patients. Arch Orthop Trauma Surg. 2018 Aug;138(8):1069-1075. doi: 10.1007/s00402-018-2941-x.
- Acar B, Kose O, Turan A, Unal M, Kati YA, Guler F. Comparison of Bioabsorbable Magnesium versus Titanium Screw Fixation for Modified Distal Chevron Osteotomy in Hallux Valgus. Biomed Res Int. 2018 Nov 19;2018:5242806. doi: 10.1155/2018/5242806.
- Choo JT, Lai SHS, Tang CQY, Thevendran G. Magnesium-based bioabsorbable screw fixation for hallux valgus surgery - A suitable alternative to metallic implants. Foot Ankle Surg. 2018 Sep 22. pii: S1268-7731(18)30392-8. doi: 10.1016/j.fas.2018.09.001.
- Klauser H. Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg. 2018 Feb 16. pii: S1268-7731(18)30030-4. doi: 10.1016/j.fas.2018.02.005.
- Gigante A, Setaro N, Rotini M, Finzi SS, Marinelli M. Intercondylar eminence fracture treated by resorbable magnesium screws osteosynthesis: A case series. Injury. 2018 Nov;49 Suppl 3:S48-S53. doi: 10.1016/j.injury.2018.09.055.
- Acar B, Unal M, Turan A, Kose O. Isolated Lateral Malleolar Fracture Treated with a Bioabsorbable Magnesium Compression Screw. Cureus. 2018;10(4):e2539. doi:10.7759/cureus.2539.
- Aktan C, Ertan MB, Turan A, Kose O. Fixation of Small Osteochondral Fragments in a Comminuted Distal Humerus Fracture with Magnesium Bioabsorbable Screws: A Case Report. Cureus. 2018 Dec 19;10(12):e3752. doi: 10.7759/cureus.3752.
Source for all fotos: Syntellix AG
Author: Ozkan KOSE, MD, MBA, FEBOT, Assoc. Prof.
Please klick here to download the CV!

Date: 08/07/2019
Source: Ozkan KOSE, MD, MBA, FEBOT, Assoc. Prof. Consultant Surgeon in Orthopaedics and Traumatology