Article: Wehrmedizinische Monatsschrift 8/2018
The Role of PSMA PET/CT and PET/MRI in Diagnosing Prostate Carcinoma
Division VI, Bundeswehr Medical Service Headquarters¹, Koblenz (Chief of Division: Oberstarzt Dr. Thomas Harbaum), Bundeswehr Institute of Preventive Medicine², Andernach (Director: Oberstarzt Prof. Dr. Dr. Dieter Leyk), Department of Nuclear Medicine³, Department of Radiology and Neuroradiology⁴, Department of Urology⁵ of the Bundeswehr Central Hospital³, Koblenz (Commander and Medical Director: Generalarzt Dr. Norbert Weller)
Manuela A. Hoffmann¹,²,³, Helmut J. Wieler³, Kerstin Smolka⁴, Hans-Ulrich Schmelz⁵, Stephan Waldeck⁴
Summary
Prostate cancer (PCa) is the leading cancer in men in Europe. The department of Urology of the Bundeswehr Central Hospital in Koblenz has founded the certified PCa-Centre Koblenz-Mittelrhein to optimise diagnostics and treatment of patients with PCa. The introduction of prostate-specific membrane-antigen (PSMA) into the diagnostic process is a worldwide breakthrough for the work of nuclear medicine departments.
The value of 68Ga-PSMA PET imaging as a diagnostic procedure for primary and recurrent PCa is assessed. The most important data from mostly retrospective studies currently available are discussed. The current knowledge on 68Ga-PSMA PET suggests that primary staging with PET/CT and/or PET/MRI is useful in patients with intermediate to high-risk PCa and that the combination with pelvic multiparametric (mp) MRI has the greatest impact on patient management. 68Ga-PSMA PET/CT is superior to 18F/11C-Choline PET/CT in primary and secondary staging. In patients with biochemical relapse, PET/CT positivity is directly associated with prostate-specific antigen (PSA) increase.
Keywords: 68Gallium-PSMA, PET/CT, PET/MRI, PET-guided personalised therapy, prostate cancer
Introduction
Prostate cancer (PCa) is the most common cancer to affect men in Germany. The estimated incidence for 2016 was 66,900 new cases. With 12,957 deaths, prostate cancer had the third highest mortality rate of all cancers in 2012 [7, 22]. Accurate diagnosis of suspected prostate carcinoma is critical when it comes to ensuring fast and curative care. In patients with presumed recurrent PCa following initial curative treatment, diagnostic imaging presents a challenge.
Diagnostic imaging of prostate carcinoma
Primary diagnosis
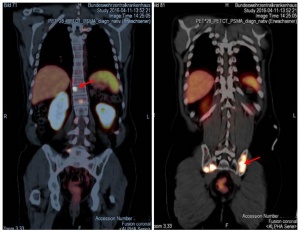 Figure 1: PSMA PET/CT scans show multiple metastases in the spine and pelvis, some of them confluent, PSMA-avid, osseous.
Figure 1: PSMA PET/CT scans show multiple metastases in the spine and pelvis, some of them confluent, PSMA-avid, osseous.
A diagnosis of PCa is still based primarily on non-specific screening methods such as PSA levels and digital rectal exams. Transrectal ultrasound-guided biopsy is often used to confirm a suspected clinical diagnosis. With this method, however, a carcinoma will remain undetected in 20–30% of cases and there is a high risk of undergrading, i.e. underestimating the aggressiveness of the tumour [5, 15, 31]. Another clinical problem is an increasing or elevated PSA level following a negative punch biopsy [14]. Recent studies have shown multiparametric MRI (mpMRI) to be superior to other diagnostic methods in patients with elevated PSA levels after negative biopsy results [5, 29, 32, 38]. The MRI protocol for the prostate usually combines thin-layered native T2-weighted sequences with diffusion-weighted sequences (DWI) followed by dynamic contrast-enhanced sequences with preferably isotropic voxels. Recent studies have shown mpMRI/ultrasound fusion-guided biopsies to achieve a significantly higher rate of detection of clinically relevant prostate malignancies than conventional diagnostic procedures [19]. There was no statistical significance in terms of differentiating between high-grade and low-grade carcinomas, however [18].
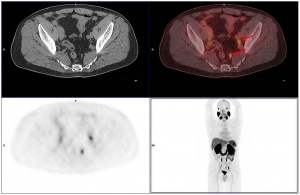 Figure 2: CT scans did not reveal any morphological abnormalities of pelvic lymph nodes which were, due to their high PSMA avidity, identified as suspicious only after the PET scan.
Figure 2: CT scans did not reveal any morphological abnormalities of pelvic lymph nodes which were, due to their high PSMA avidity, identified as suspicious only after the PET scan.
There is growing evidence in the relevant literature that using PSMA PET helps further increase the reliability of diagnosis. PSMA is an integral membrane protein that, in prostate carcinoma cells, is expressed at levels up to 1,000 times that of normal prostate cells. PSMA expression is particularly elevated in high-grade carcinomas in patients with metastatic cancer and hormone-refractory prostate cancer (figure 1). As a result, PSMA has become a topic of great interest in the context of specific imaging and treatment of PCa. Working groups in Heidelberg [4, 10] and Munich [10] deserve credit for having introduced a diagnostic method that is now internationally recognised.
Expression of PSMA in the prostate increases with the aggressiveness of the tumour, whereas it is barely detectable in benign prostate tissue [8, 34, 37]. That is why hybrid methods are currently being developed to perform PSMA PET and mpMRI in a single-step exam in order to combine the high sensitivity of MRI with the metabolic assessment provided by PET [23, 26, 36]. This is particularly important with regard to undergrading of tumours as well as when it comes to optimising the TNM classification before treatment. In most cases, 68-Ga PSMA radiotracers are used and more recently F-18 PSMA radiotracers [35].
Diagnosing recurrence
Around 40% of patients who undergo local treatment with a primarily curative intent experience a recurrence of disease [30]. Most of these patients develop a biochemical recurrence, which means they have elevated PSA levels without showing any clinical evidence of metastases [9]. Biochemical tumour recurrence occurs in about 20–30% of patients after radical prostatectomy and in up to 60% after radiotherapy [12, 24]. In the event of such recurrence, standard sectional imaging with spiral CT or MRI is often of limited use, in particular when it comes to assessing pathologic lymph nodes. That is why substantial effort has gone into improving available imaging methods. In recent years, the relevant research has mainly focused on PSMA.
The current German S3 Guideline on diagnosing PCa reflects this development and PSMA PET/CT is now an integral part of the diagnostic process [27, 28].
Significance of PSMA PET/CT
Since the introduction of the substance and method, a great number of publications have confirmed their clinical importance. In their study of traditional morphologic imaging in patients before radiotherapy, Giesel et al. [13] found that applying morphological CT criteria, mainly based on the size and shape of the lymph nodes, resulted in only 22% of PSMA-positive lesions being classified as suspicious for malignancy. In a comparison between PSMA PET/CT and 3D volumetric CT, two thirds of the patients were upstaged from cN0 to cN1. Previous studies had shown that high signal-to-background ratios are typical for PSMA PET/CT [2, 4, 25]. The study by GIESEL et al. [13] also demonstrated that the smallest PET-positive lesion, in this case a lymph node, had a short axis diameter of only 2.4 mm. This is almost the limit of what is physically feasible (1.7 mm) with this procedure. Especially for patients to be treated with salvage radiotherapy, PSMA PET/CT is of great significance. Salvage radiotherapy is recommended for patients with PSA levels < 0.5 ng/ml [16, 27]. PSMA PET/CT has been shown to help detect pathological contrast agent enhancement beyond the prostatic fossa even with such low PSA values. The most common locations were pelvic lymph nodes in about half of the patients with a positive PET/CT scan (figure 2). This would mean a change in treatment plan for about a quarter of all patients with low PSA levels referred for PSMA PET/CT [1, 11]. PSMA PET/CT will thus have an impact on patient management, helping to avoid, for example, ineffective radiotherapy and its sometimes considerable side effects or irradiation using radiation fields that include the affected lymph nodes. Recent studies have also shown 68Ga-PSMA PET/CT to be superior to 18F-Choline PET/CT in terms of image quality, higher specificity and good correlation of PSMA images with the Gleason score [17, 20].
Magnetic resonance imaging, and in particular multiparametric highfield 3T MRI, is currently the most sensitive morphological imaging method available on the market. Combining the high sensitivity of MRI with a highly specific tracer stands to reason. A direct comparison between PSMA PET/CT and PET/multiparametric MRI is therefore of considerable interest with regard to future studies [6]. It seems certain that, going forth, PET/CT and PET/MRI hybrid imaging systems will deliver better results than stand-alone morphological imaging [33].
Our studies comparing PSMA PET/CT and PET/MRI
Once PET/MRI had been introduced at the Bundeswehr Central Hospital in Koblenz, the findings in the first patients examined using both methods were compared.
Testing methods
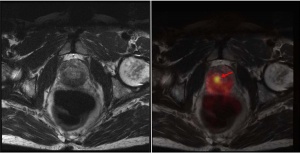 Figure 3: PSMA PET/MRI scans show right-sided prostate cancer focus with considerably increased PSMA avidity.
Figure 3: PSMA PET/MRI scans show right-sided prostate cancer focus with considerably increased PSMA avidity.
PET/CT images were produced with the Siemens Biograph 64 True Point PET/CT scanner (TrueV HD). Full-body scans were conducted 60 minutes after 68Ga-PSMA-11 injection (median 176 MBq, range 157–268 MBq). We received PSMA-11 from the Department of Nuclear Medicine at the University Medical Centre in Mainz and Advanced Accelerator Applications (AAA) in Bonn. PET/MRI scans with simultaneous acquisition of MRI sequences (non-contrast enhanced) were produced about 2 hours after injection using the Siemens 3 Tesla Biograph mMR hybrid system, immediately after PET/CT. The following sequences were used in the PET/MRI scan: transverse, coronal and sagittal T2 Turbo Spin Echo (TSE); coronal T1 TSE; sagittal T2; diffusion-weighted sequences (B50/500/1000); transverse short tau inversion recovery (STIR).
Results
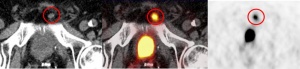 Figure 4: PSMA PET/CT (fused image: middle) of left-sided infrapubic lymph node of 9 x 8 mm on non-contrast-enhanced CT (left) with considerably increased PSMA avidity observed on PET (right).
Figure 4: PSMA PET/CT (fused image: middle) of left-sided infrapubic lymph node of 9 x 8 mm on non-contrast-enhanced CT (left) with considerably increased PSMA avidity observed on PET (right).
A total of 193 PSMA PET/CT scans have been performed at the Department of Nuclear Medicine of the Bundeswehr Central Hospital in Koblenz since July 2015. Once PET/MRI had been established for standard use at the Bundeswehr Central Hospital in Koblenz, we were able to refer a first series of 34 patients for PET/MRI immediately following PET/CT (figure 3).
In this small group of patients, both types of scan revealed similar findings in 31 cases (31/34), with no additional pathological abnormality (lesion/lymph node/local recurrence) found in the second scan but not the first.
In three cases (3/34), there was a discrepancy between the two examinations; PET/CT was superior in two cases and PET/MRI in one case.
In one patient, three prostate cancer foci with a high level of metabolic activity observed in PET/CT were not clearly identifiable in PET/MRI and intraprostatic PET/MRI findings were not assessable.
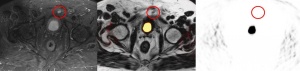 Figure 5: PSMA PET/MRI (fused image: middle) of left-sided infrapubic lymph node (9 x 8 mm) hyperintense on T2-weighted MRI image (left, transverse STIR sequence) without evidence of increased PSMA avidity on PET (right).
Figure 5: PSMA PET/MRI (fused image: middle) of left-sided infrapubic lymph node (9 x 8 mm) hyperintense on T2-weighted MRI image (left, transverse STIR sequence) without evidence of increased PSMA avidity on PET (right).
In a second case, a small left-sided infrapubic lymph node of 9 x 8 mm with considerably increased PSMA avidity observed in PET/CT could not be verified by PET/MRI due to halo artefacts. With an increased standardised uptake value (SUV) of 10.1 detected on the PET/CT scan, the lymph node was clearly suspicious for malignancy (figure 4). T2-weighted MRI showed hyperintensity of the lymph node, while PET/MRI did not reveal an increased PSMA avidity level due to extensive halo artefacts (figure 5). In the literature, halo artefacts are described as typical artefacts on PET/MRI that affect the detection of malign lesions located near the urinary bladder or kidneys [3, 21].
In the third case, the PET/MRI scan revealed four lymphogenic metastases, whereas PET/CT identified only two lymph node metastases.
Conclusions and outlook
Additional PET/MRI scans are important for our colleagues with a view to ensuring quality and confirming findings and we intend to conduct a prospective study to compare both methods, especially as there is currently little data available on this issue. The study should include newer algorithms for scatter corrections in PET/MRI to avoid halo artefacts.
Key statements
- mpMRI/ultrasound fusion-guided biopsies achieve significantly higher detection rates of clinically relevant prostate malignancies than conventional diagnostic procedures.
- The introduction of prostate-specific membrane antigen (PSMA) has been a global breakthrough in clinical diagnosis and treatment in nuclear medicine departments.
- PSMA PET/CT is considered an integral part of diagnosing recurrent PCa.
- PSMA PET and mpMRI complement each other in terms of sensitivity and specificity.
Literature
- Afshar-Oromieh A, Avtzi E, Giesel FL et al.: The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42 (2): 197 - 209.
- Afshar-Oromieh A, Zechmann CM, Malcher A et al.: Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2014; 41 (1): 11 - 20.
- Afshar-Oromieh A, Haberkorn U, Schlemmer HP et al.: Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: Initial experience. Eur J Nucl Med Mol Imaging 2014; 41 (5): 887 - 897.
- Afshar-Oromieh A, Malcher A, Eder M et al.: PET imaging with a 68Gagallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013; 40 (4): 486 - 495.
- Ahmed HU, El-Shater Bosaily A, Brown LC et al.: Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. The Lancet 2017; 389 (10071): 815 - 822.
- Avanesov M, Karul M, Derlin T: (68)Ga-PSMA als neuer Tracer für die Evaluation des Prostatakarzinoms: Vergleich zwischen PET-CT und PET-MRT beim biochemischen Rezidiv. Radiologe 2015; 55 (2): 89 - 91.
- Böhmer D, Wirth M, Miller K et al.: Radiotherapy and hormone treatment in prostate cancer. Dtsch Arztebl Int 2016; 113 (14): 235 - 241.
- Ceci F, Uprimny C, Nilica B et al.: (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: Which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging 2015; 42 (8): 1284 - 1294.
- Cornford P, Bellmunt J, Bolla M et al.: EAU-ESTRO-SIOG Guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 2017; 71 (4): 630 - 642.
- Eder M, Schäfer M, Bauder-Wüst U et al.: 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012; 23 (4): 688 - 697.
- Eiber M, Maurer T, Souvatzoglou M et al.: Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015; 56 (5): 668 - 674.
- Freedland SJ, Presti JC, Amling CL et al.: Time trends in biochemical recurrence after radical prostatectomy: Results of the SEARCH database. Urology 2003; 61 (4): 736 - 741.
- Giesel FL, Fiedler H, Stefanova M et al.: PSMA PET/CT with Glu-urea-Lys-(Ahx)-68Ga(HBED-CC) versus 3D CT volumetric lymph node assessment in recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42 (12): 1794 - 1800.
- Grisanti S, Antonelli A, Buglione M et al.: Analysis of circulating tumor cells in prostate cancer patients at PSA recurrence and review of the literature. Anticancer Res 2016; 36 (6): 2975 - 2981.
- Heidegger I, Skradski V, Steiner E et al.: High risk of under-grading and -staging in prostate cancer patients eligible for active surveillance. PLoS ONE 2015; 10 (2): e0115537.
- Heidenreich A, Bastian PJ, Bellmunt J et al.: EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014; 65 (2): 467 - 479.
- Hoffmann MA, Wieler HJ, Maus S et al.: Diagnostic performance of 68Ga-PSMA PET/CT to detect significant prostate cancer and comparison with 18F-Choline PET/CT. J Nucl Med 2017; 58 Supplement 1: 540.
- Hoffmann MA, Wieler HJ, Jakobs FM et al.: The diagnostic significance of multiparametric MRI fusion-guided biopsy of the prostate to detect clinically significant prostate carcinoma in patients with elevated PSA levels and negative punch biopsy results. Korrelation mit dem Gleason Score. Nuklearmedizin 2017; 56 (4): 147 - 155.
- Hoffmann MA, Taymoorian K, Ruf C et al.: Diagnostic performance of multiparametric magnetic resonance imaging and fusion targeted biopsy to detect significant prostate cancer. Anticancer Res 2017; 37 (12): 6871 - 6877.
- Hoffmann MA, Miederer M, Wieler HJ et al.: Diagnostic performance of 68Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with18FEC PET/CT. Oncotarget 2017; 8 (67): 111073 - 111083.
- Hoffmann MA, Wieler HJ, Smolka K: „Head-to-head comparison of 68Ga-PSMA PET/CT and 68Ga-PSMA PET/MRI for restaging of biochemical recurrent prostate cancer. J Transl Sci; (im Druck).
- Kaatsch P, Spix C, Katalinic A et al.: Beiträge zur Gesundheitsberichterstattung des Bundes – Krebs in Deutschland 2011/2012. Berlin: Robert Koch-Institut 2015.
- Kesch C, Visensia M, Radtke JP: Intraindividueller Vergleich von 18F-PSMA-PET/CT, mpMRT und radikalem Prostatektomiepräparat bei Männern mit primär diagnostiziertem Prostatakarzinom. Nuklearmedizin 2017; 56 (4): V54.
- Khuntia D, Reddy CA, Mahadevan A et al.: Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1-T3 prostate carcinoma in the prostate-specific antigen era: What should we expect? Cancer 2004; 100 (6): 1283 - 1292.
- Krohn T, Verburg FA, Pufe T et al.: (68)GaPSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: An important pitfall in clinical practice. Eur J Nucl Med Mol Imaging 2015; 42 (2): 210 - 214.
- Langer DL, van der Kwast TH, Evans AJ et al.: Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2--sparse versus dense cancers. Radiology 2008; 249 (3): 900 - 908.
- Leitlinienprogramm Onkologie. Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF: Interdisziplinäre Leitlinie der Qualität S3 zur Früherkenung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 4.0.
http://leitlinienprogramm-onkologie.de/uploads/tx_sbdownloader/LL_Prostata_Langversion_4.0.pdf (last accessed on 03 May 2018). - Lenzen-Schulte M: Leitlinienprogramm Onkologie: Eine „living guideline“ zum Prostatakarzinom. Dtsch Arztebl Int 2017; 114 (3): A80.
- Mottet N, Bellmunt J, Bolla M et al.: EAU-ESTRO-SIOG Guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71 (4): 618 - 629.
- Mullins JK, Feng Z, Trock BJ et al.: The impact of anatomical radical retropubic prostatectomy on cancer control: The 30-year anniversary. J Urol 2012; 188 (6): 2219 - 2224.
- Oliveira IS, Pontes-Junior J, Abe DK et al.: Undergrading and understaging in patients with clinically insignificant prostate cancer who underwent radical prostatectomy. Int Braz J Urol 2010; 36 (3): 292 - 299.
- Pepe P, Garufi A, Priolo GD et al.: Multiparametric MRI/TRUS fusion prostate biopsy: Advantages of a transperineal approach. Anticancer Res 2017; 37 (6): 3291 - 3294.
- Picchio M, Mapelli P, Panebianco V et al.: Imaging biomarkers in prostate cancer: Role of PET/CT and MRI. Eur J Nucl Med Mol Imaging 2015; 42 (4): 644 - 655.
- Sweat SD, Pacelli A, Murphy GP et al.: Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998; 52 (4): 637 - 640.
- Szabo Z, Mena E, Rowe SP et al.: Initial Evaluation of (18)FDCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol 2015; 17 (4): 565 - 574.
- Woythal N, Kempkensteffen C, Miller K: Ga-68 PSMA PET/CT in the detection of primary prostate cancer: an initial experience. Nuklearmedizin 2017; 56: V48.
- Wright GL, Haley C, Beckett ML et al.: Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol 1995; 1 (1): 18 - 28.
- Wu X, Reinikainen P, Kapanen M et al.: Diffusion-weighted MRI provides a Useful biomarker for evaluation of radiotherapy efficacy in patients with prostate cancer. Anticancer Res 2017; 37 (9): 5027 - 5032.
Image source (all figures): Bundeswehr Central Hospital, Koblenz
Citation mode:
Hoffmann MA, Wieler HJ, Smolka K, Schmelz HU, Waldeck S: PSMA PET/CT and PET/MRI in prostate carcinoma diagnosis Wehrmedizinische Monatsschrift 2018; 62(8): 266 - 270.
For the authors:
Oberfeldarzt Dr. med. Manuela A. Hoffmann
Kommando Sanitätsdienst der Bundeswehr, Koblenz
Institut für Präventivmedizin der Bundeswehr, Andernach
E-Mail: manuela1hoffmann@bundeswehr.org
Date: 10/15/2018